Atomic Theory
History
Beginning in about 600 B.C., many Greek philosophers struggled to understand the nature of matter. Some said everything was made of water, which comes in three forms (solid ice, liquid water, and gaseous steam). Others believed that matter was made entirely of fire in everchanging forms. Still others believed that whatever comprised matter, it must be something that could not be destroyed but only recombined into new forms. If they could see small enough things, they would find that the same "building blocks" they started with were still there. One of these philosophers was named Democritus. He imagined starting with a large piece of matter and gradually cutting it into smaller and smaller pieces, finally reaching the smallest piece. This tiniest building block that could no longer be cut he named atomos, Greek for "no-cut." Atomos has been changed in modern times to "atom." The atoms Democritus envisioned differed only in shape and size. In his theory, different objects looked different because of the way the atoms were arranged. Aristotle, one of the most influential philosophers of that time, believed in some kind of "smallest part" of matter but not with Democritus's descriptions. Aristotle said there were only four elements (earth, air, fire, water) and that these had some smallest unit that made up all matter. Aristotle's teachings against the idea of Democritus's atom were so powerful that the idea of the atom fell out of philosophical fashion for the next 2,000 years.
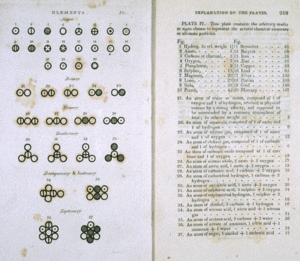
Although atomic theory was abandoned for this long period, scientific experimentation, especially in chemistry, flourished. From the Middle Ages (C. 1100) onward, many chemical reactions were studied. By the seventeenth century, some of these chemists began thinking about the reactions they were seeing in terms of smallest parts. They even began using the word atom again. One of the most famous chemists of the end of the eighteenth century was Antoine Lavoisier. His chemical experiments involved very careful weighing of all the chemicals. He reacted various substances until they were in their simplest state. He found two important factors: (1) the simplest substances, which he called elements, could not be broken down any further, and (2) these elements always reacted with each other in the same proportions. The same more complex substances he called compounds. For example, two volumes of hydrogen reacted exactly with one volume of oxygen to produce water. Water could be broken down to always give exactly two volumes of hydrogen and one volume of oxygen. Lavoisier had no explanation for these amazingly consistent results. However, his numerous and careful measurements provided the clue to another chemist named John Dalton.
Dalton realized that if elements were made up of atoms, a different atom for each different element, atomic theory could explain Lavoisier's results. If two atoms of hydrogen always combined with one atom of oxygen, the resulting combination of atoms, called a molecule, would be water. Dalton published his explanation in 1803. This year is considered the beginning of modern atomic theory. Scientific experiments that followed Dalton were attempts to characterize how many elements there were, what the atoms of each element were like, how the atoms of each element were the same and how they differed, and, ultimately, whether there was anything smaller than an atom.
Additional topics
Science EncyclopediaScience & Philosophy: A-series and B-series to Ballistic Missiles - Categories Of Ballistic MissileAtomic Theory - History, Describing Characteristics Of Atoms, Applications Of Atomic Theory