Chemical Reactions
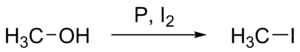
Chemical reactions describe the changes between reactants (the initial substances that enter into the reaction) and products (the final substances that are present at the end of the reaction). Describing interactions among chemical species, chemical reactions involve a rearrangement of the atoms in reactants to form products with new structures in such a way as to conserve atoms. Chemical equations are notations that are used to concisely summarize and convey information regarding chemical reactions.
In a balanced chemical reaction all of the matter (i.e., atoms or molecules) that enter into a reaction must be accounted for in the products of a reaction. Accordingly, associated with the symbols for the reactants and products are numbers (stoichiometry coefficients) that represent the number of molecules, formula units, or moles of a particular reactant or product. Reactants and products are separated by addition symbols (addition signs). The addition signs represent the interaction of the reactants and are used to separate and list the products formed. The chemical equations for some reactions may have a lone reactant or a single product. The subscript numbers associated with the chemical formula designating individual reactants and products represent the number of atoms of each element that are in each molecule (for covalently bonded substances) or formula unit (for ironically associated substances) of reactants or products.
For a chemical reaction to be balanced, all of the atoms present in molecules or formula units or moles of reactants to the left of the equation arrow must be present in the molecules, formula units and moles of product to the right of the equation arrow. The combinations of the atoms may change (indeed, this is what chemical reactions do) but the number of atoms present in reactants must equal the number of atoms present in products.
Charge is also conserved in balanced chemical reactions and therefore there is a conservation of electrical charge between reactants and products.
Although chemical equations are usually concerned only with reactants and products chemical reactions may proceed through multiple intermediate steps. In such multi-step reactions the products of one reaction become the reactants (intermediary products) for the next step in the reaction sequence.
Reaction catalysts are chemical species that alter the energy requirements of reactions and thereby alter the speed at which reactions run (i.e., control the rate of formation of products).
Combustion reactions are those where oxygen combines with another compound to form water and carbon dioxide. The equations for these reactions usually designate that the reaction is exothermic (heat producing). Synthesis reactions occur when two or more simple compounds combine to form a more complicated compound. Decomposition reactions reflect the reversal of synthesis reactions (e.g., reactions where complex molecules are broken down into simpler molecules). The electrolysis of water to make oxygen and hydrogen is an excellent example of a decomposition reaction.
Equations for single displacement reactions, double displacement, and acid-base reactions reflect the appropriate reallocation of atoms in the products.
In accord with the laws of thermodynamics, all chemical reactions change the energy state of the reactants. The change in energy results from changes in the in the number and strengths of chemical bonds as the reaction proceeds. The heat of reaction is defined as the quantity of heat evolved or absorbed during a chemical reaction. A reaction is called exothermic if heat is released or given off during a chemical transformation. Alternatively, in an endothermic reaction, heat is absorbed in transforming reactants to products. In endothermic reactions, heat energy must be supplied to the system in order for a reaction to occur and the heat content of the products is larger than that of the reactants. For example, if a mixture of gaseous hydrogen and oxygen is ignited, water is formed and heat energy is given off. The chemical reaction is an exothermic reaction and the heat content of the product(s) is lower than that for the reactants. The study of energy utilization in chemical reactions is called chemical kinetics and is important in understanding chemical transformations.
A chemical reaction takes place in a vessel which can be treated as a system. If the heat "flows" into the vessel during reaction, the reaction is said to be "endothermic" (e.g., a decomposition process) and the amount of heat, say, q, provided to the system is taken as a positive quantity. On the other hand, when the system has lost heat to the outside world, the reaction is "exothermic" (e.g., a combustion process) and q is viewed as a negative number. Normally the heat change involved in a reaction can be measured in an adiabatic bomb calorimeter. The reaction is initiated inside a constant-volume container. The observed change in temperature and the information on the total heat capacity of the colorimeter are employed to calculate q. If the heat of reaction is obtained for both the products and reactants at the same temperature after reaction and also in their standard states, it is then defined as the "standard heat of reaction," denoted by ΔH°.
Both chemical kinetics and thermodynamics are crucial issues in studying chemical reactions. Chemical kinetics help us search for the factors that influence the rate of reaction. It provides us with the information about how fast the chemical reaction will take place and about what the sequence of individual chemical events is to produce observed reactions. Very often, a single reaction like A B may take several steps to complete. In other words, a chain reaction mechanism is actually involved which can include initiation, propagation, and termination stages, and their individual reaction rates may be very different. With a search for actual reaction mechanisms, the expression for overall reaction rate can be given correctly. As to determining the maximum extent to which a chemical reaction can proceed and how much heat will be absorbed or liberated, we need to estimate from thermodynamics data. Therefore, kinetic and thermodynamic information is extremely important for reactor design.
As an example of a chemical reaction, Hydrogen (H2) and oxygen (O2) gases under certain conditions can react to form water (H2O). Water then exists as solid (ice), liquid, or vapor (steam); they all have the same composition, H2O, but exhibit a difference in how H2O molecules are brought together due to variations in temperature and pressure.
Chemical reactions can take place in one phase alone and are termed "homogeneous." They can also proceed in the presence of at least two phases, such as reduction of iron ore to iron and steel, which are normally described as "heterogeneous" reactions. Quite frequently, the rate of chemical reaction is altered by foreign materials, so-called catalysts, that are neither reactants nor products. Although usually used to accelerate reactions, reaction catalysts can either accelerate or hinder the reaction process. Typical examples are found in Pt as the catalyst for oxidation of sulfur dioxide (SO2) and iron promoted with Al2O3 and K as the catalyst for ammonia (NH3) synthesis.
Chemical reactions can characterized as irreversible, reversible, or oscillating. In the former case, the equilibrium for the reaction highly favors formation of the products, and only a very small amount of reactants remains in the system at equilibrium. In contrast to this, a reversible reaction allows for appreciable quantities of all reactants and products co-existing at equilibrium. H2O + 3NO2 &NA; 2HNO3 + NO is an example of a reversible reaction. In an oscillating chemical reaction, the concentrations of the reactants and products change with time in a periodic or quasi-periodic manner. Chemical oscillators exhibit chaotic behavior, in which concentrations of products and the course of a reaction depend on the initial conditions of the reaction.
Chemical reactions may proceed as a single reaction A → B, series reactions A → B → C, side-by-side parallel reactions A → B and C → D, two competitive parallel reactions B and A C, or mixed parallel and series reactions A + B → C and C + B → D. In order for chemical reactions to occur, reactive species have to first encounter each other so that they can exchange atoms or groups of atoms. In gas phases, this step relies on collision, whereas in liquid and solid phases, diffusion process (mass transfer) plays a key role. However, even reactive species do encounter each other, and certain energy state changes are required to surmount the energy barrier for the reaction. Normally, this minimum energy requirement (e.g., used to break old chemical bonds and to form new ones) is varied with temperature, pressure, the use of catalysts, etc. In other words, the rate of chemical reaction depends heavily on encounter rates or frequencies and energy availability, and it can vary from a value approaching infinity to essentially zero.
See also Catalyst and catalysis; Chemical bond; Chemistry; Conservation laws; Entropy; Enzyme; Equation, chemical; Equilibrium, chemical; Molecular formula; Moles; Stereochemistry.
Resources
Books
Housecroft, Catherine E., et al. Inorganic Chemistry. Prentice Hall, 2001.
Incropera, Frank P., and David P. DeWitt. Fundamentals of Heat and Mass Transfer. 5th ed. John Wiley & Sons, 2001.
Moran, Michael J., and Howard N. Shapiro. Fundamentals of Engineering Thermodynamics. 4th ed. John Wiley & Sons, 2000.
K. Lee Lerner Pang-Jen Kung
Additional topics
- Chemical Warfare - Antipersonnel Agents—chemicals Used Against People, Use Of Herbicides During The Vietnam War, Use Of Petroleum As A Weapon During The Gulf War
- Chemical Oxygen Demand
- Other Free Encyclopedias
Science EncyclopediaScience & Philosophy: Categorical judgement to Chimaera