Osmosis (Cellular)
Osmosis In Plant Cells
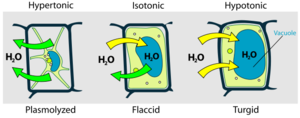
Plant cells are surrounded by rigid cellulose walls, (unlike animal cells), but plant cells still take in water by osmosis when placed in pure water. However, plant cells do not burst because their cellulose cell walls limit how much water can move in. The cell walls exert pressure, called turgor pressure, as the cells take up water. Turgor pressure is analogous to the air pressure of an inflated tire. These physical forces can be described by a simple mathematical equation: ψ = P + π, where ψ ("Psi") is the water potential, a measure of the overall tendency of water to move into a cell; P is the pressure potential, a measure of the turgor pressure exerted by the cell walls; and π is the osmotic potential (see above). Water always moves from regions of higher ψ to areas of lower ψ. Animal cells do not have cellulose cell walls, so P = 0 and ψ = π for these cells.
The significance of osmosis to plant function is best appreciated by describing its role in the regulation of guard cells. Guard cells are specialized cells scattered across the surface of plant leaves. Each pair of guard cells surround a special pore, termed a stoma (plural stomata), and control its opening. Guard cells have a special arrangement of microfibrils in their walls, so that when the guard cells swell the stomata open. When the stomata of a plant leaf are open, this increases photosynthetic gas exchange and movement of water out of the plant by transpiration.
In many plants, certain environmental stimuli, such as sunlight, stimulate the guard cells to take up potassium from surrounding cells. This causes their osmotic potential (π) to decrease and water moves in by osmosis. Thus, the guard cells swell, the stomata open, and the rate of gas exchange through the stomata increases. This increases the rate of photosynthesis and plant growth.
Other environmental cues, such as water shortage, cause plants to transmit chemical signals to the guard cells, causing them to release potassium, which increases their osmotic potential, and to lose water by osmosis. This causes the guard cells to shrink, so closing the stomata, and decreasing the rate of water transpiration through the stomata. This reduces water loss and prevents wilting of the plant.
Plants rely upon other environmental signals to regulate the osmotic movement of water into their guard cells and the opening of the stomata, so that the advantage of increased photosynthesis is balanced against the disadvantage of increased water loss.
See also Diffusion.
Resources
Books
Salisbury, F. B., and C. W. Ross. Plant Physiology. Belmont, CA: Wadsworth Publishing Company, 1992.
Peter A. Ensminger
Additional topics
Science EncyclopediaScience & Philosophy: Octadecanoate to OvenbirdsOsmosis (Cellular) - Osmosis In Red Blood Cells, Osmosis In Plant Cells