Atomic Models
The Modern Atomic Model
The development of quantum mechanics served as the foundation of the modern atomic theory. In 1922, the American physicist Arthur H. Compton (1892–1962) conduced x-ray scattering experiments that confirmed and advanced Einstein's theory on the dual nature of light. In 1923, the French physicist Louis-Victor de Broglie (1892–1987) expanded on this theory by proposing that all matter, as well as radiation, behaves both as a particle and a wave. Until this time, scientists had viewed matter and energy as distinct phenomena that followed different laws.
Broglie's proposal was not supported by experimental or mathematical evidence until 1926 when the Austrian physicist Erwin Schrödinger (1887–1961) developed his mathematical wave equation. Schrödinger proposed that electrons also behaved like waves. His wave equation could be used to find the frequency of the electrons and then Planck's equation could be used to find the corresponding energy. Schrödinger's equation gave a much more precise description of an electron's location and energy than Bohr's model could. It could also be used for atoms with more than one electron. Furthermore, only waves of specific frequencies could be solved using his equation. This demonstrated that only certain energies are possible for the electrons in an atom. Further experiments demonstrated that Broglie was correct in his assertion that matter could behave as waves, as electrons were diffracted and exhibited interference.
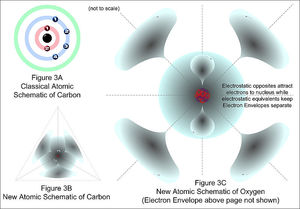
In 1927, German physicist Werner Heisenberg (1901–1976) developed what is now known as the Heisenberg uncertainty principle. This hypothesis states that the position and velocity of an electron, or any moving particle, cannot both be known at the same time. This meant that the solutions to the Schrödinger wave equation, known as wave functions, could describe only the probability of finding an electron in a given orbit. Therefore, the electrons are not located in discrete orbits, as hypothesized in the Bohr model, but instead occupy a hazier region, called an orbital. An orbital indicates a probable location of the electrons in an atom instead of a definite path that they follow. The probable location of the electrons in an orbital is described by a series of numbers called quantum numbers.
The quantum model of the atom uses four quantum numbers to describe the arrangement of electrons in an atom, much like an address describes the locations of houses on a street. This arrangement is known as the electron configuration. The atoms for each element have their own distinct electron configuration. The ground state electron configuration of an atom represents the lowest energy arrangement of the electrons in an atom. The placement of electrons in a particular configuration is based on three principles. The first, the Aufbau principle, states that an electron will occupy the lowest possible energy orbital available. The Pauli exclusion principle states that each electron in an atom has its own distinct set of four quantum numbers. No two electrons in an atom will have the same set.
Lastly, Hund's rule states that even though each orbital can hold two electrons, the electrons will occupy the orbitals such that there are a maximum number of orbitals with only one electron. Developed by the German scientist, Friedrich Hund (1896–1997), Hund's rule allows scientists to predict the order in which electrons fill an atom's suborbital shells.
Hund's rule is based on the Aufbau principle that electrons are added to the lowest available energy level (shell) of an atom. Around each atomic nucleus, electrons occupy energy levels termed shells. Each shell has a spherical s orbital and, starting with the second shell, orbitals (p, d, f, etc.) and suborbitals (e.g., 2px,2py, 2pz) with differing size, shapes and orientation (i.e., direction in space).
Although each suborbital can hold two electrons, the electrons all carry negative charges and, because like charges repel, electrons repel each other. In accord with Hund's rule, electrons space themselves as far apart as possible by occupying all available vacant suborbitals before pairing up with another electron. The unpaired electrons all have the same spin quantum number (represented in electron configuration diagrams with arrows all pointing either upward or downward).
In accord with the Pauli exclusion principle that states that each electron must have its own unique set of quantum numbers that specify its energy and because all electrons have a spin of 1/2, each suborbital can hold up to two electrons only if their spins are paired +1/2 with - 1/2. In electron configuration diagrams, paired electrons with opposite spins are represented by paired arrows pointing up and down.
Although Hund's rule accurately predicts the electron configuration of most elements, exceptions exist, especially when atoms and ions have the opportunity to gain additional stability by having filled s shells or half-filled d or f orbitals.
In 1928, the English physicist P.A.M. Dirac (1902–1984) formulated a new equation to describe the electron. Schrödinger's equation did not allow for the principles of relativity, and could only be used to describe movement of particles that are slower than the speed of light. Because electrons move at a much greater velocity, Dirac introduced four new wave functions to describe the behavior of electrons. These functions described electrons in various states. Two of the states corresponded to their spin orientations in the atom, but the other two could not be explained. In 1932, the American physicist Carl David Anderson (1905–1991) discovered the positron, which explained the two mystery states described by Dirac.
Modern physics has expanded the atomic model by introducing new particles that can be created in vacuum tubes. Such particles are called antiparticles, because they can be "destroyed" or converted into other forms of energy. Antiparticles include positrons, muons, pions, hadrons, baryons, mesons, and quarks. These particles can combine with each other or split to form new and different particles. For example, three quarks can combine to form a proton, a neutron, or a baryon. Many of these particles have such high energies, they have never actually been observed in the laboratory. The quantum field theory is the study of the behavior of these antiparticles and how they relate to the three subatomic particles (the proton, neutron, and electron). According to the quantum field theory, the atom can be subdivided not only into protons, neutrons, and electrons, but into antiparticles as well.
See also Atomic spectroscopy; Atomic weight; Electromagnetic spectrum; Particle detectors; Quantum mechanics.
Resources
Books
Chattergee, Lali. The Exotic Lifestyles of Subatomic Particles Dubuque, OH: Kendall/Hunt Publishers, 2000.
Davis and Metcalf. Modern Chemistry. New York: HBJ School, 2000.
Gribbin, John R. and Mary Gribbin. Q is for Quantum: An Encyclopedia of Particle Physics New York: Free Press, 2000.
LaMay, H. Eugene. Chemistry: Connections to Our Changing World. Upper Saddle River, NJ: Prentice Hall, 2000.
Myers, Oldham, and Tocci. Chemistry: Visualizing Matter. New York: Holt Rinehart & Winston, 2000.
Other
Particle Data Group. Lawrence Berkeley National Laboratory. "The Particle Adventure: The Fundamentals of Matter and
Force" [cited February 5, 2003]. <http://particleadventure.org/particleadventure/>.
K. Lee Lerner
Jennifer McGrath
Additional topics
Science EncyclopediaScience & Philosophy: A-series and B-series to Ballistic Missiles - Categories Of Ballistic MissileAtomic Models - Early Atomic Theory, Discovery Of The Electron, The First Atomic Models, Discovery Of The Proton