All About Atoms
Take a Closer Look, Large and Small, Atomic Numbers, Mass Numbers, Electron Shells
So if everything is made of atoms, what exactly is an atom? What is it like, and what is it made of?
Take a Closer Look
Atoms may be small, but they are made up of parts that are even smaller. At the center is the nucleus. The nucleus is made of protons and neutrons.
Atoms also have other parts called electrons. The electrons whirl around the nucleus very quickly.
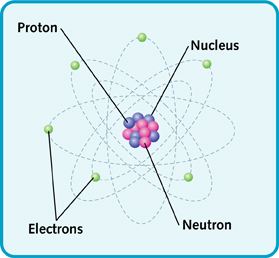 The main parts of an atom.
The main parts of an atom.
Large and Small
There are many different types of atoms. They are all tiny, but some are bigger than others.
For example, a helium atom is a small atom. It has a nucleus made of two protons and two neutrons. It has two electrons zooming around the nucleus. A carbon atom is bigger. It has a nucleus made of six protons and six neutrons. It also has six electrons.
Many atoms are even bigger than this, but they all have the same basic shape and parts.
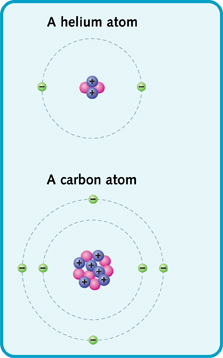 Simple diagrams of helium and carbon.
Simple diagrams of helium and carbon.
 Oxygen atoms are another type of atom. They are found in the air we breathe.
Oxygen atoms are another type of atom. They are found in the air we breathe.
Atomic Numbers
Scientists need to label the different types of atoms, so they know which is which. For this reason, they give atoms numbers.
These are known as atomic numbers. The atomic number depends on how many protons an atom has. For example, oxygen has eight protons. So its atomic number is 8.
 This scientist is looking at a model of a group of different types of atoms.
This scientist is looking at a model of a group of different types of atoms.
Mass Numbers
An atom also has a mass number. This is the number of protons and neutrons added together. An oxygen atom has eight protons and eight neutrons. That makes sixteen. So its mass number is 16.
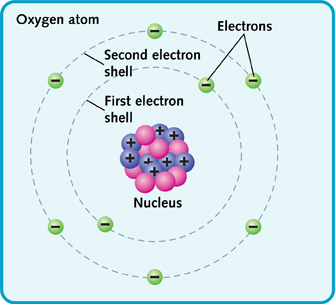 The first shell holds two electrons. The second shell has space for up to eight electrons. In an oxygen atom, it holds six electrons.
The first shell holds two electrons. The second shell has space for up to eight electrons. In an oxygen atom, it holds six electrons.
Electron Shells
The number of electrons in an atom is important, too. The electrons move around in different layers, called electron shells.
The first shell is closest to the nucleus. It holds up to two electrons. The second shell can hold up to eight electrons. There can be more shells, too. An atom has as many shells as it needs to hold all its electrons.
How Small Are Atoms?
It's hard to imagine how small atoms are. One single atom is about one 250-millionth of an inch (0.000000004 inches). That's the same as one ten-millionth of a millimeter across (0.0000001 mm).
Counting Atoms
You could fit more than twenty million atoms into one period on this page. The page itself is about a million atoms thick. If you lined up atoms in a row, you would need fifty million of them to make a line as long as your little fingernail.
EMPTY SPACE
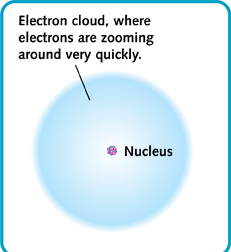
Most of the matter in an atom is in its nucleus. The nucleus takes up a tiny part of the atom. The rest is the space around the nucleus, where the electrons whirl. So an atom is mostly empty space. That means that all the things around us—and our own bodies—are also mostly empty space!
 Gold leaf is one of the thinnest materials you can find. It's about 300 atoms thick. Here it covers the domed roofs of these beautiful towers in Moscow, Russia.
Gold leaf is one of the thinnest materials you can find. It's about 300 atoms thick. Here it covers the domed roofs of these beautiful towers in Moscow, Russia.
The Story of Atoms
Atoms are far too tiny for us to see with just our eyes. So how do we know they are there?
Ancient Greek scientists wondered what things were made of. Some said everything was made of water or air. But one scientist, Democritus, said that everything was made of tiny particles. The particles did not change, and they could not be broken. He named these particles atoms. The word atom is Greek for “thing that cannot be split in two.”
John Dalton
The scientist John Dalton lived from 1766 to 1844. He found out much more about atoms. He thought that different materials must be made up of different types of atoms. Different atoms must have different numbers of parts. Dalton is often called “the father of chemistry.” Chemistry is the study of what materials are made of and how they react to each other.
 A portrait of John Dalton.
A portrait of John Dalton.
DEMOCRITUS
Democritus lived in ancient Greece from about 460 to 370 B.C. He was a philosopher. A philosopher is someone who thinks about and tries to understand the world. He studied many things besides atoms. These included animals, plants, weather, and stars.
Under the Microscope
Today, we have very strong microscopes. With them, we can actually see atoms. What we can see proves that Democritus and Dalton were right.
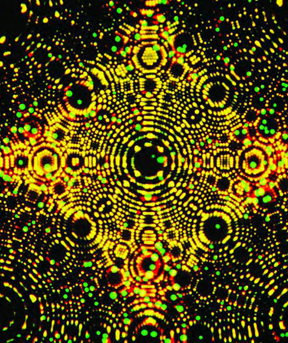 The tiny dots in this picture show where iridium atoms are, or have just been. They are seen under a special microscope.
The tiny dots in this picture show where iridium atoms are, or have just been. They are seen under a special microscope.
Additional topics
- Amazing Elements - Think of an Element, The Elements, Important Elements, Code Names
- Introducing Atoms and Molecules - What Are Atoms and Molecules?, Matter, The Way Things Are, Differences
- Other Free Encyclopedias