Particle Theory
Properties of Solids, Liquids, and Gases, Solids, Liquids, Gases
Properties of Solids, Liquids, and Gases
All materials are made of tiny particles. These are called atoms. Sometimes atoms join together to form molecules. Scientists talk about different materials by talking about these particles. They talk about how the atoms (or molecules) are arranged. This helps scientists explain solids, liquids, and gases.
Solids
In solids, the particles are arranged in a regular pattern. They are held together by strong forces. This is why solids have a fixed shape. You cannot squash or change the volume of solids. The particles are already as close to each other as they can get.
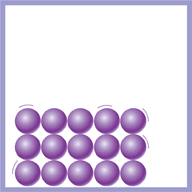 This diagram shows the particles in a solid.
This diagram shows the particles in a solid.
Liquids
With liquids, the particles are still very close. But the forces holding them together are not as strong as in solids. The particles can move around. This is why you can pour a liquid. You cannot squash a liquid because the particles are still very close together.
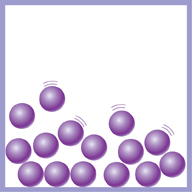 This diagram shows the particles in a liquid.
This diagram shows the particles in a liquid.
Gases
The particles in a gas are a long way apart. They move around very quickly. Gases are easy to squash because there is a lot of space between the particles.
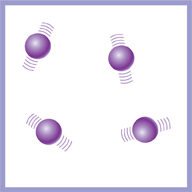 This diagram shows the particles in a gas.
This diagram shows the particles in a gas.
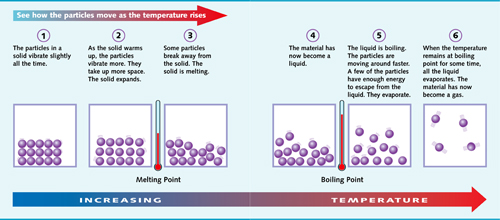
What Happens When a Material is Heated?
This diagram shows what happens to the particles of a solid material as it is heated.
Additional topics
- Ice: a Special Solid? - Why Does Ice Float?, Icebergs, Breaking Up Rocks, What Will Happen If the Ice Melts?
- Volcanoes and Lava - What Happens to Lava When It Freezes?, Danger, Volcano!
- Other Free Encyclopedias